Product Parameters
Dengue fever IgG IgM Blood Test is a qualitative test for the detection of IgM and IgG antibodies to dengue virus in human serum, plasma or whole blood. This test is for in-vitro diagnostic use only. 
Feature
Product Name | Dengue IgM/ IgG Test Kit |
Format | cassette |
Type | Pathological Analysis Equipments |
Specimen | Serum/Plasma/Whole Blood |
Methodology | Colloidal Gold |
Certificate | CE ISO |
OEM | Acceptable |
Packaging | Bag+Box+Carton |
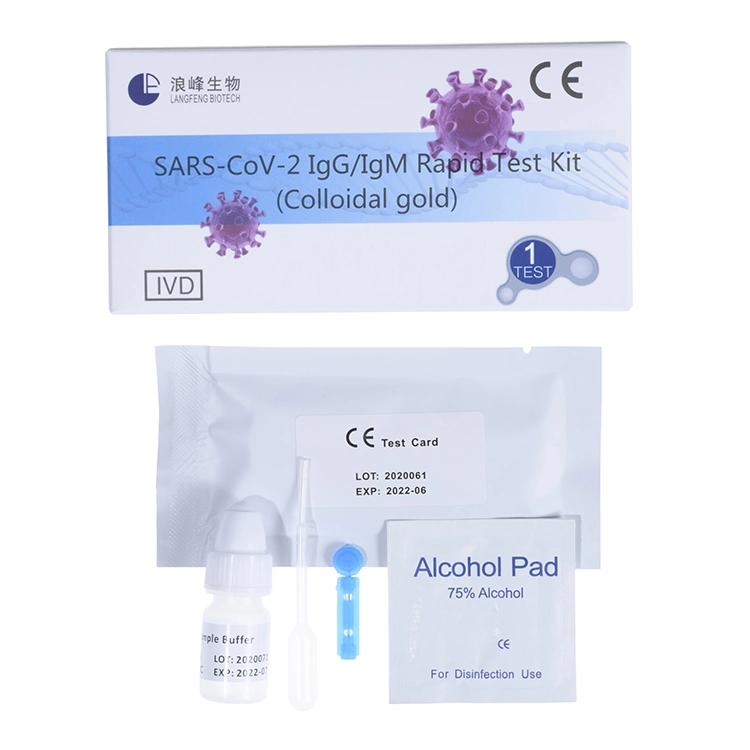
Technical Sheet:
| Fast Reaction | 10-15 minutes |
| Packing | 40Test/Box |
| Carton Size | 63*35*30.5CM / Carton |
| Gross Weight | 9.5KG/Carton |
| MOQ | 25 Boxes / Carton /1000pcs |
| After-sale Service | Return and Replacement |
| Certificate: | TUV and CE |
Chinese Tips against virus war :
1. Stay at home, do not go out or go to the place where there are many people.
2. When you need to go out, please wear mask. When you enter into the elevator or into your car, please wear gloves, and not to press
the button or pull the car door with your fingers directly without protection.
3. When you get back home, please spray alcohol or disinfectant to your hands and shoes.
4. Wash your hands frequently.
5. When you are at home, pls use alcohol pads to rub your phones to kill virus very often.
6. Please keep warm and do not catch a cold.
7. Go to bed early and get up early to strengthen immunity from diseases.



Procedure and result reading
For Strip Format:
1) remove the strip from the poly pouch and pay it on a flat surface.
2) For whole blood specimen :Allow 2 hanging drops of finger stick whole blood specimen to fall into the center of the sample pad on
the strip, then add 1 drop of buffer and start the timer.For serum or plasma specimens: Using the provided disposable pipette,
transfer 3 drops of specimen (approximately 75 μ L) to the sample pad of the strip and start the timer.
3) Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20minutes.


2. Negative result may occur when detecting short-term infected samples or window period samples, indicate that the specific antibodies of MP does not exist or the concentration is below detection limit.
3. The results of the reagent are only for clinical reference, which is not the only basis for clinical diagnosis and treatment. A confirmed diagnosis and treatment should only be made by a physician after all clinical and laboratory findings have been evaluated.
4. Positive results of the patients who used to receive blood transfusions or other blood products therapy,should be analyzed cautiously.
5. Abnormal results may occur according to operator error or drug use. If Mycoplasma pneumonia is still suspected, a sample should be collected later and tested again.
When can I get the price?
We usually quote within 24 hours after getting your inquiry.
If you are urgent to get the price, pls call or email us so that we give you the quotation in time.
How can I get a sample to check your quality?
After price confirmation, you can require samples to check the quality.
Can you do the design for us?
Yes, we have professional designers with rich experience. Just tell us your ideas and we will make the items on your request. It does not matter if you do not have someone to complete the files. Send us high resolution images, your logo & test files, then tell us how you would like to arrange them. We will send you the finished files for confirmation at last.
What about the lead time for mass production?
To be honest, it depends on the order quantity and the production arrangements of our factory.
Under normal circumstances, we can control lead time within a week.
We suggest that you start inquiry one month before the date you would like to get the products in your country.
What is your terms of delivery?
We accept EXW, FOB, CFR, CIF etc. You can choose one terms which is the most convenient or effective.